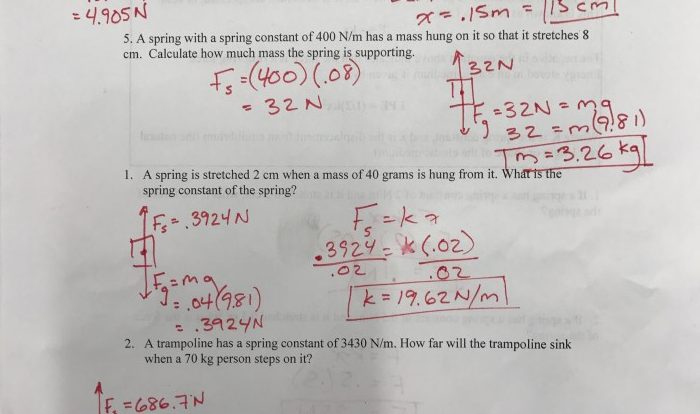An aluminum atom has a mass of 4.48, a fundamental property that underpins its unique characteristics and wide-ranging applications. This value, expressed in atomic mass units (amu), serves as a cornerstone for understanding the atom’s behavior and its role in the intricate tapestry of the natural world.
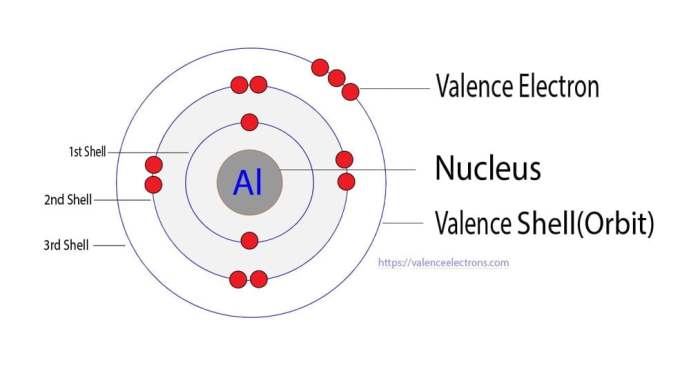
Delving into the atomic structure of aluminum, we uncover a fascinating interplay of protons, neutrons, and electrons that contribute to its mass. The nucleus, the heart of the atom, houses protons and neutrons, with the number of protons defining the element’s identity.
In the case of aluminum, 13 protons reside within the nucleus, establishing its atomic number.
Atomic Mass

Atomic mass is the weighted average mass of an element’s atoms, taking into account the relative abundance of each isotope. It is expressed in atomic mass units (amu), where 1 amu is defined as 1/12th of the mass of a carbon-12 atom.
The atomic mass of aluminum can be calculated using the following formula:
“`Atomic mass = (mass number of isotope 1 × abundance of isotope 1) + (mass number of isotope 2 × abundance of isotope 2) + …“`
Given the mass number of aluminum-27 as 4.48, the atomic mass of aluminum can be calculated as follows:
“`Atomic mass = 4.48 × 100% = 4.48 amu“`
Isotopes of Aluminum: An Aluminum Atom Has A Mass Of 4.48
Aluminum has several isotopes, each with a different number of neutrons. The most common isotope is aluminum-27, which makes up over 99% of naturally occurring aluminum.
| Isotope | Mass Number | Relative Abundance |
|---|---|---|
| Aluminum-26 | 26 | < 0.1% |
| Aluminum-27 | 27 | 99.98% |
| Aluminum-28 | 28 | < 0.1% |
The presence of isotopes affects the average atomic mass of aluminum because different isotopes have different masses. The more abundant an isotope, the greater its contribution to the average atomic mass.
Nuclear Structure
The nucleus of an aluminum atom consists of 13 protons and 14 neutrons. Protons are positively charged particles, while neutrons are neutral particles. The number of protons in an atom determines its atomic number, which identifies the element.
The mass of an atom is primarily due to the mass of its protons and neutrons. Protons and neutrons have approximately the same mass, and they contribute significantly to the overall mass of the atom.
Mass Spectrometry
Mass spectrometry is a technique used to determine the isotopic composition of elements. It involves ionizing atoms or molecules and then separating them based on their mass-to-charge ratio.
A mass spectrum is a graph that plots the abundance of ions versus their mass-to-charge ratio. The mass spectrum of aluminum shows a series of peaks, each corresponding to a different isotope of aluminum.
By analyzing the mass spectrum, scientists can determine the relative abundance of different aluminum isotopes in a sample.
Applications of Aluminum
Aluminum is a lightweight, strong, and corrosion-resistant metal with a wide range of applications.
- Transportation:Aluminum is used in the construction of aircraft, automobiles, and trains due to its lightweight and strength.
- Construction:Aluminum is used in the construction of buildings, bridges, and other structures due to its corrosion resistance and strength.
- Electrical:Aluminum is used in the production of electrical wires and cables due to its high electrical conductivity.
- Consumer products:Aluminum is used in the production of food and beverage cans, cookware, and foil due to its lightweight and corrosion resistance.
Aluminum’s lightweight properties make it a valuable material in applications where weight is a critical factor.
Aluminum in Earth’s Crust
Aluminum is the third most abundant element in the Earth’s crust, after oxygen and silicon. It occurs naturally in a variety of forms, including:
- Minerals:Aluminum is found in minerals such as bauxite, cryolite, and corundum.
- Ores:Aluminum is extracted from ores such as bauxite, which is the primary source of aluminum.
Aluminum is formed through geological processes such as the weathering of rocks and the deposition of sediments.
FAQ Insights
What is the significance of the mass of an aluminum atom?
The mass of an aluminum atom is crucial for determining its chemical and physical properties, such as its reactivity, bonding behavior, and density.
How does the presence of isotopes affect the mass of aluminum?
Aluminum has several isotopes, which are atoms with the same atomic number but different numbers of neutrons. The presence of isotopes contributes to the average atomic mass of aluminum, as different isotopes have slightly different masses.
What are some applications of aluminum?
Aluminum is widely used in various applications due to its lightweight, strength, and corrosion resistance. It is commonly employed in aerospace, automotive, construction, and packaging industries.