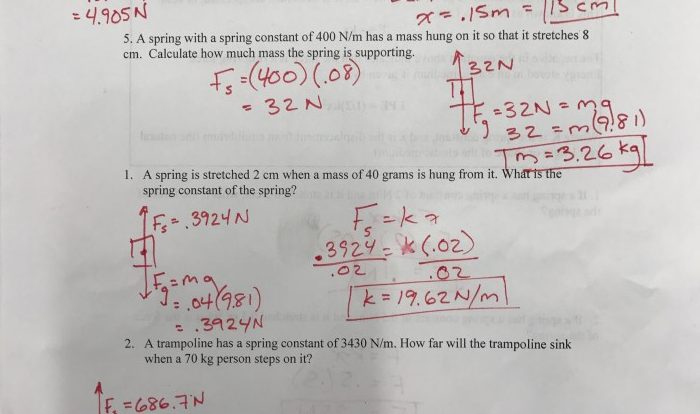Embark on an exploration of the heating curve of water worksheet, a fundamental tool for understanding the thermal behavior of water. This worksheet provides a comprehensive overview of the concept, its significance, and practical applications in various scientific fields.
Delving into the different stages of the heating curve, from solid to liquid to gas phases, we uncover the factors that influence its shape and characteristics. Pressure, impurities, and dissolved gases all play a role in shaping the curve’s behavior, offering insights into the complex thermal properties of water.
Overview of Heating Curve of Water
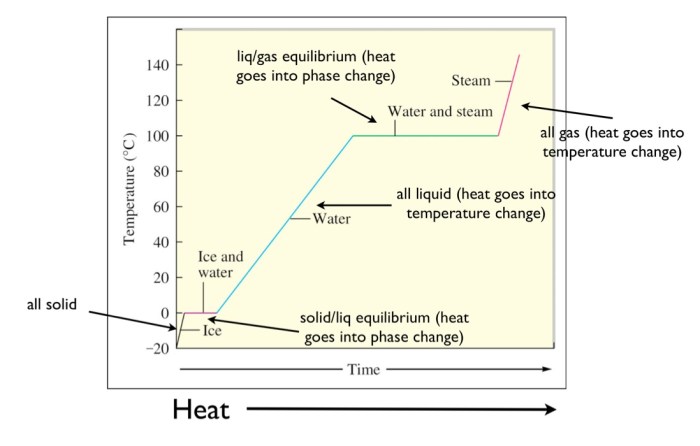
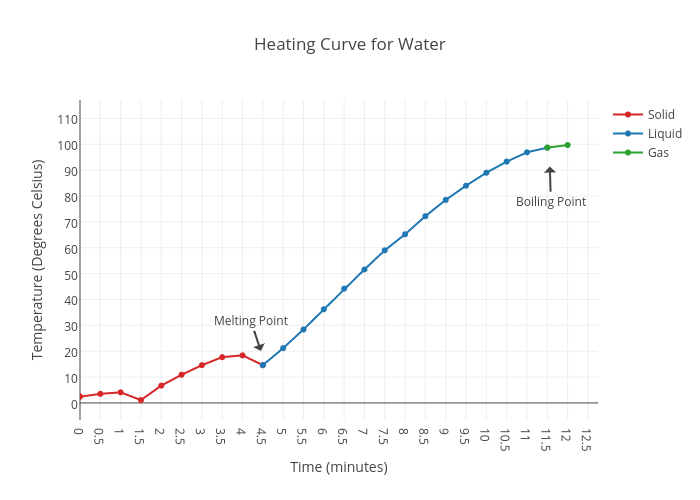
A heating curve graphically depicts the temperature change of a substance as it is heated. It provides insights into the thermal behavior and phase transitions of the substance. The heating curve of water exhibits distinct stages corresponding to the solid, liquid, and gas phases.
Solid Phase
As solid water (ice) is heated, its temperature rises steadily until it reaches its melting point, 0 °C (32 °F). At this point, the temperature remains constant as the ice melts, absorbing energy to overcome intermolecular forces and transition to the liquid phase.
Liquid Phase
Once the ice has completely melted, the temperature of the liquid water continues to rise linearly with the addition of heat. During this stage, the water molecules gain kinetic energy, increasing their motion and causing the temperature to increase.
Gas Phase
When the liquid water reaches its boiling point, 100 °C (212 °F), it undergoes a phase transition to the gas phase. Similar to the melting process, the temperature remains constant during boiling as the water absorbs energy to overcome intermolecular forces and transition to the gaseous state.
Factors Affecting the Heating Curve
The shape and characteristics of the heating curve of water can be influenced by several factors, including pressure, impurities, and dissolved gases. Understanding these factors is crucial for accurately interpreting the curve and predicting the behavior of water under various conditions.
Pressure
Pressure affects the boiling point of water. As pressure increases, the boiling point also increases. This is because higher pressure makes it more difficult for water molecules to escape into the vapor phase. Consequently, the heating curve will shift to the right, indicating a higher boiling point at a given pressure.
Impurities
The presence of impurities, such as salts or dissolved solids, can alter the heating curve of water. Impurities can act as nucleation sites for water molecules, facilitating the formation of vapor bubbles and lowering the boiling point. As a result, the heating curve will shift to the left, indicating a lower boiling point in the presence of impurities.
Dissolved Gases
Dissolved gases, such as oxygen or nitrogen, can also influence the heating curve of water. Dissolved gases can form bubbles within the water, which can act as nucleation sites and lower the boiling point. The extent to which dissolved gases affect the heating curve depends on the concentration and type of gas present.
Applications of the Heating Curve
The heating curve of water has various practical applications in different fields:
In chemistry, the heating curve can be used to determine the specific heat capacity of water. The specific heat capacity is the amount of heat energy required to raise the temperature of one gram of a substance by one degree Celsius.
By measuring the slope of the heating curve, the specific heat capacity of water can be calculated.
Engineering Applications
In engineering, the heating curve of water is used in the design of heating and cooling systems. By understanding the thermal properties of water, engineers can design systems that efficiently transfer heat energy.
Physics Applications
In physics, the heating curve of water is used to study the phase transitions of water. Phase transitions are changes in the physical state of a substance, such as from solid to liquid or liquid to gas. By studying the heating curve, physicists can gain insights into the thermodynamics of phase transitions.
Designing a Heating Curve Experiment: Heating Curve Of Water Worksheet
Designing a heating curve experiment for water involves setting up the apparatus, collecting data, and analyzing the results. Here’s a step-by-step guide to help you conduct the experiment efficiently and accurately.
Apparatus Setup
- Gather the necessary materials: a calorimeter, a thermometer, a heat source (e.g., Bunsen burner), a stirrer, and a graduated cylinder.
- Fill the calorimeter with a known mass of water (e.g., 100 g).
- Place the thermometer in the water and secure it with a rubber stopper.
- Set up the heat source under the calorimeter and adjust the flame to a low setting.
Data Collection, Heating curve of water worksheet
- Start the timer and record the initial temperature of the water.
- Gently heat the water while stirring continuously to ensure uniform temperature distribution.
- Record the temperature of the water at regular intervals (e.g., every 30 seconds).
- Continue heating until the water reaches its boiling point (100°C).
Data Analysis
- Plot the temperature of the water against time to create a heating curve.
- Identify the different stages of the heating curve: initial heating, plateau (latent heat of fusion), and final heating.
- Calculate the specific heat capacity of water from the slope of the initial heating and final heating segments.
- Determine the latent heat of fusion of water from the plateau region.
Troubleshooting Common Issues
Heating curve experiments can encounter various challenges and errors that may affect the accuracy of data collection. Identifying and addressing these issues is crucial to ensure reliable results.
Temperature Measurement Errors
- Incorrect calibration:Ensure the thermometer or temperature sensor is properly calibrated before the experiment.
- Faulty equipment:Replace or repair faulty thermometers or temperature sensors that provide inaccurate readings.
- Heat loss:Minimize heat loss by using insulated containers and avoiding drafts.
Heating Rate Issues
- Inconsistent heating:Maintain a constant heating rate throughout the experiment by using a heating mantle or hot plate with adjustable temperature settings.
- Too fast heating:Slow down the heating rate to avoid overshooting the desired temperature or creating temperature gradients within the sample.
- Too slow heating:Increase the heating rate to prevent prolonged heating times that may affect the sample’s stability.
Data Collection and Interpretation Errors
- Incorrect data logging:Use a reliable data logger or computer software to accurately record temperature readings.
- Misinterpretation of heating curve:Consult literature or reference materials to correctly interpret the heating curve and identify phase transitions and thermal events.
- Insufficient data points:Collect sufficient data points throughout the heating process to ensure accurate representation of the heating curve.
Essential FAQs
What is the significance of the heating curve of water?
The heating curve of water provides insights into the thermal behavior of water, including its phase transitions and the energy changes associated with these transitions.
How can the heating curve be used to determine water properties?
The heating curve can be used to determine properties such as specific heat capacity and enthalpy of fusion, which are important for understanding the thermal behavior of water.
What factors can affect the shape of the heating curve?
Factors such as pressure, impurities, and dissolved gases can influence the shape and characteristics of the heating curve.